Covalent bonds form
as atoms seek to obtain a complete set of electrons in their outer electron shells by sharing electrons with another atom..
For example, oxygen contains two vacancies in its outer shell, and can therefore
form two covalent bonds. Hydrogen can form one covalent bond, having one spot
available in its outer shell. Thus, in an H20 molecule, oxygen shares
an electron pair with each of two hydrogens, filling outer shell electron vacancies
for all three atoms.
Covalent bonds are the strongest
bonds that link atoms, with energies of bond formation
on the order of -50 to -100 kcal/mole. The merging of electron orbitals that
occurs during covalent bond formation places constraints on the angles at which
atoms can be linked because orbital merging is energetically favorable only
in certain Directions Please leave comments/suggestions or please acknowledge use of this site by visiting our feedback page When two atoms share two
pairs of electrons, the atoms are said to share a double bond. Double bonds
are stronger than single bonds, with the result that double-bonded atoms are
held closer together than those engaged in a single bond. Atoms in a double
bond are not able to freely rotate about the bond axis as in a single bond,
because sharing two electrons in their outer shells places additional constraints
on the ways that their electron clouds can be oriented.
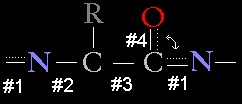
The peptide bond that links
amino acids together in a protein chain is special in that it has the characteristics
of a partial double bond. This is because the electrons shared in a double bond
between the carboxy carbon and oxygen
of one amino acid can resonate (delocalize) and can participate in
the peptide bond linking the carboxy carbon to the
amino nitrogen of the next amino acid.
 |
Thus, bonds 1 and 4, above, show a partial double bond character, whereas bonds 2 and 3 are standard single bonds. The partial double bond nature of the peptide bond has profound consequences for protein structure since the alpha carbons in a protein backbone can potentially rotate more freely around their bond axes (bonds 2 and 3, above) than the backbone atoms of the peptide bond.