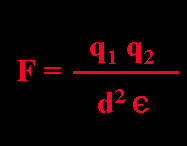 |
F (bond strength); q (charge); d (distance); ε (dielectric constant of solvent)
Ionic bonds are electrostatic attractions between oppositely charged atoms. The strength of an ionic bond is related to distance: ionic bonds are not "broken" - their strength is simply diminished by distance. Ionic bonds may be formed at any angle, since electrons are not shared covalently. Thus, variable arrangements of ionic bonds are found in different contexts. The strength (force) of an ionic bond is given by:
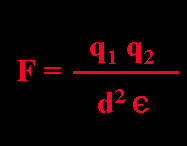 |
F (bond strength); q (charge); d (distance); ε (dielectric constant of solvent)
Experiments show that bond strength (F) is directly proportional to the amount of charge on each interacting atom (q1, q2), is inversely proportional to the square of the distance (d) between them, and is inversely related to ε, the dielectric constant of solvent. The value of the dielectric constant (a measure of the capacity of solvent molecules to neutralize charge by reorientation) is correlated with solvent polarity.
Due to the inverse relationship between ionic bond strength and ε, the local environment of an ionic bond largely determines its strength. If an ionic bond is surrounded by polar H2O molecules (ε=85), the charges of the interacting molecules are effectively reduced by interaction with polar H2O shells, and the strength of the bond is correspondingly reduced. However, if an ionic bond is inaccessible to solvent, as in the interior of a protein, the charged species are mostly surrounded by hydrophobic side chains with low ε's. For example, for CH3, ε=1. Thus, ionic bonds that are not accessible to solvent (H2O) tend to be quite strong, even approaching that of a covalent bond (>50 kcal/mole).