I. Subunit structure
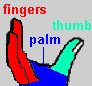
The enzyme reverse transcriptase (RT) is used by retroviruses to transcribe their single-stranded RNA genome into single-stranded DNA and to subsequently construct a complementary strand of DNA, providing a DNA double helix capable of integration into host cell chromosomes. Functional HIV1-RT is a heterodimer containing subunits of 66 kDa (p66) and 51 kDa (p51). p66 contains two the two domains responsible for the two catalytic activities of RT, the N-terminal polymerase domain and the C-terminal RNase H domain. The polymerase domain catalyzes polymerization of DNA in a primer strand complementary to a template strand of RNA (see below). The RNase H domain catalyzes the degradation of the RNA template (see below). p51 is processed by proteolytic cleavage of p66 and corresponds to the polymerase domain of the p66 subunit. The polymerase domain of p66 includes three subdomains that can be described as the fingers, palm, and thumb of a clasping right hand:
The hand subdomains serve to clasp the RNA-DNA duplex in the process of RNA directed DNA polymerization. The fingers and thumb domains are the walls of a nucleic acid binding cleft, with the palm subdomain seving as a base containing the DNA polymerase active site (see below). The connection subdomain connects the hand subdomains of the polymerase domain and the RNase H domain of p66, which provides the ribonuclease activity of HIV-RT, digesting the RNA template after a DNA copy is polymerized. The p66 palm and connection subdomains contain three-stranded beta sheets with alpha helices on one side. The thumb subdomain comprises three alpha helices. Interestingly, although p66 and p51 are identical in their primary amino acid sequence (except for length) and they share similar subdomain structures, they are topologically quite distinct. For example, three catalytic, aspartate residues from the palm subdomain are exposed in the nucleic acid binding cleft of p66, but are buried in p51, which lacks a discernable cleft. Another striking difference between the two subunits is the orientation of the connection subdomain; in p51 it is tucked into a central position and contacts all of the other subdomains, but in p66 it contacts only RNase H and the thumb. A question arises as to why HIV has evolved a heterodimer in which the smaller subunit (p51) is a cleavage product of the larger. One speculation (e.g. Kohlstaedt, et al., 1992) is that the selection for streamlined genomes in retroviruses has forced the evolution of different protein subunits encoded by the same gene. In the case of HIV-RT, subunits with different structural and functional properties can be produced by proteolytic cleavage of one of two initially identical subunits.
return to beginning of the exhibit II. Nucleic Acid-RT Interactions
HIV-1 RT contains an ~60 Angstrom groove between the polymerase and RNase active sites. The connection subdomains of both p66 and p51 form the floor of this groove. The template-primer nucleic acid duplex fits into this groove and is cradled by the hand subdomains of p66, which form a nucleic acid binding cleft that includes the polymerase active site (see below). Numerous residues from the fingers, palm, thumb, and connection subdomains and the RNase H domain contact the nucleic acid backbone. Residues from one helix of the thumb subdomain contact bases directly. The few, direct contacts between the nitrogenous bases of the template-primer double helix and RT residues are mostly van der Waals interactions. These include minor groove base interactions with thumb residues of helix H, palm residues near the primer strand 3' terminus, and an RNase H domain residue. Hydrogen bonding between tyr183 and a G in the minor groove is observed. Focusing now on the polymerase active site, the incoming nucleotide (in this case, dTTP) is positioned to be added to the growing primer strand. Nucleophillic attack on the alpha phosphate of the incoming nucleotide by the 3' oxygen on the 3' carbon of the primer terminus will produce the covalent linkage of the new nucleotide to the primer strand The two remaining phosphates of dTTP will form a leaving group. The dTTP is positioned by hydrogen bonding with a complementary base in the template strand, and by interactions with Mg++ ions and residues of the palm and fingers subdomains. Three catalytic aspartate residues from the palm subdomain are involved in coordinating the Mg++ ions. In a well studied, two-metal mechanism found in numerous other polymerases, these ions serve two key functions: 1) stabilizing the ionized form of the 3' oxygen (O-), increasing its nucleophilicity and leading to the attack on the alpha phosphate; 2) stabilizing negative charges on the diphosphate leaving group. * At left are the superimposed protein backbones of unliganded HIV-1 RT (Rodgers, et al., 1995) and the liganded form of the enzyme (Huang, et al., 1998), bound to a double helical, nucleic acid, template-primer substrate (not shown). Although the backbones are largely congruent in the RNAase H domains and the connection subdomains of p51 and p66, the arrangement of the hand subdomains of p66 changes upon binding nucleic acid. These changes are readily observed by viewing the two forms of the enzyme sequentially. Template-primer binding causes the fingers subdomain to curl toward the palm in the liganded enzyme, relative to the unliganded. The polymerase cleft can be seen to widen in this conformation, with the thumb subdomain of the liganded form opening to accomodate the nucleic acid. These conformational adjustments to nucleic acid binding appropriately position the polymerase active site residues for catalysis, as discussed above. *This composite PDB file was produced by combining 1RTD and 1HMV.
return to beginning of the exhibit III. HIV RT Inhibitors
Because of the importance of RT to HIV replication, inhibitors of this enzyme are potential theraputic agents in the battle against HIV. One class of RT inhibitors is the nucleoside analogs like AZT (= zidovudine, Retrovir), ddI, ddC, and d4T. At left is shown a normal nucleotide DNA precursor, CTP, and the RT inhibitor, AZT. AZT, like other dideoxy nucleoside analogs, lacks a 3' oxygen on the ribose sugar, having a nitrogen linkage instead. Incorporation of AZT into a primer strand of DNA causes RT to cease DNA polymerization because there is no 3' oxygen to attack an incoming nucleotide's 5' alpha phosphate (see above).
Another class of compounds that inhibit HIV-RT are the non-nucleoside inhibitors (NNIs). These inhibitors (e.g., APA) have been shown to bind in a pocket formed between two beta sheets of the p66 palm, ~10 Angstroms away from the polymerase active site aspartates (e.g. Ding, et al., 1995). The internal surface of this pocket is predominantly hydrophobic, being constructed primarily from leucine, valine, tryptophan and tyrosine residues. Although the NNIs are chemically diverse compounds, the crystal structures (e.g., Ren et al., 1995) reveal a common mode of binding. Each compound has a unique structure accomodated by plasticity in regions of the surrounding protein to allow some unfavourable contacts to be relieved without changing the overall binding mode. Depending on the NNI bound, the volume of the pocket varies between ~600 and ~700 Angstroms3, with the inhibitors occupying ~250-350 Angstroms3. There is a clear matching of NNI shape to fit in this volume and in some cases this is achieved by conformational rearrangement of the compound from its lowest energy structure in solution. These results provide some understanding of the structural basis of the potency of the inhibitors and may suggest possible modifications that could improve interactions with the enzyme.
return to beginning of the exhibit IV. ReferencesHuang, H., Chopra, R., Verdine, G.L., Harrison, S.C. (1998). Structure of a covalently trapped catalytic complex of HIV-1 reverse transcriptase: implications for drug resistance. Science 282: 1669-1675. Ding, J., Das, K., Tantillo, C., Zhang, W., Clark Jr., A.D., Jessen, S., Lu, X., Hsiou, Y., Jacobo-Molina, A., Andries, K., et al. (1995 ). Structure of HIV-1 reverse transcriptase in a complex with the non-nucleoside inhibitor alpha-APA R 95845 at 2.8 A resolution. Structure 3: 365-379. Kohlstaedt, L.A., Wang, J., Friedman, J.M., Rice, P.A., and Steitz, T.A. (1992). Crystal Structure at 3.5 Å Resolution of HIV-1 Reverse Transcriptase Complexed with an inhibitor. Science 256: 1783-1790 Ren, J., Esnouf, R., Garman, E., Somers, D., Ross, C., Kirby, I., Keeling, J., Darby, G., Jones, Y., Stuart, D. (1995). High resolution structures of HIV-1 RT from four RT-inhibitor complexes. Nat.Struct.Biol. 2: 293-302. Rodgers, D.W., Gamblin, S.J., Harris, B.A., Ray, S., Culp, J.S., Hellmig, B., Woolf, D.J., Debouck, C., Harrison, S.C. (1995). The structure of unliganded reverse transcriptase from the human immunodeficiency virus type 1. PNAS 92: 1222-1226. return to beginning of the exhibit
|