Brian Smith
CLU/AMGEN
Physical Science
July ‘03
Chemical Bonding in Solids
In a Nutshell …
Valence electrons are
responsible for bonding between atoms and determine whether atoms will form
ionic, covalent or metallic bonds. The
properties of the combination of atoms are the result of the bonds between
them.
Covalent bonds form
between atoms of similar electronegativity by sharing electrons between the
atoms. The sharing of electrons enables
each atom to fill its outer electron shell (octet rule). This results in a
strong attractive force between atoms; the covalent bond.
Atoms having a significant
difference in electronegativity transfer electrons to form ionic bonds. The more electronegative atom (electron
acceptor) accepts one or more valence electrons from the less electronegative
atom (electron donor). The acceptor
fills its outer electron shell by adding to it and the donor ends up with a
full outer electron shell (previously the filled shell beneath the partially-filled
outer shell) by donating the electrons in its unfilled outer shell. The electron donor forms a positively charged
particle (cation) and the acceptor becomes negatively charged (an anion). The electrostatic force between the cation
& anion is the ionic bond.
In metals, valence electrons are delocalized. That is, they are very loosely connected to the nucleus to which they “belong” and may be some distance from that nucleus. As a result, they are able to move freely and metals are therefore good conductors of heat & electricity as well as being malleable & ductile.
Place in the Curriculum:
Chemical bonding will follow a thorough study of atomic structure, electron configuration and the Periodic Table.
Central
Concepts:
1. Valence
electrons determine an atom’s chemical properties.
2. Atoms
combine to reduce their overall potential energy.
3. The nature of the bond is determined by the number of valence
electrons each atom needs to gain or lose.
a. Metals form metallic bonds in which valence electrons are
delocalized.
b. A metal and a non-metal will donate and accept, respectively, one
or more electrons to form an ionic bond.
As a result, each will have a filled outer electron shell; a noble gas
configuration.
c. Non-metals
will combine by sharing electrons, equally or unequally (polar or non-polar),
to form a covalent bond. Each will
usually have a filled outer electron shell containing its own electrons and
those it shares with other atoms.
Objectives:
1. Determine the number of valence electrons in an atom from its
position in the periodic table.
2. Draw Lewis-dot structures of the representative elements.
3. State and apply the octet (duet) rule.
4. Describe the role of noble gas electron configurations in ion
formation.
5. Describe the formation of cations and anions using orbital diagrams,
Lewis-dot formulas, and standard ion formulas.
6. State the characteristics of an ionic bond and recognize compounds
having ionic bonds.
7. Relate the properties of ionic substances to ionic bonding.
8. Explain the electrical conductivity of melted and of aqueous
solutions of ionic compounds.
9. Describe the metallic bond in terms of vacant valence orbitals and
loosely-bound valence electrons. Relate
some properties of metals to the type of bonding present.
10. Describe the
formation of a covalent bond between two nonmetallic elements.
Activities:
1. Demonstrations
a. Electrical Conductivity of Solutions
… see page 1
b. Magnetic Analogy for Bonding Forces
… see page 2
c. Force at a Distance … see page 3
2. Labs
a. Physical Properties and Chemical Bonding in
Solids … see page 4
b. Metals and Ionic Crystals
… see page 5
c. Combining Cations & Anions Using
Model Ions … see page 6
3. Key Questions … see page 7
Assessment:
1. Rubric for Teacher Observation & Evaluation … see page 8 … under development
2. Rubrics for Lab Write-ups
… see page 9 … under development
3. Written Quizzes & Tests
… see page 10 … under development
page 1
Electrical Conductivity of
Solutions
Purpose:
This demonstration provides experimental evidence
on the nature of ionic and molecular substances in solution.
Materials:
·
Electrical conductivity apparatus
(commercial or home-made)
·
6-8 beakers, 250 mL
·
Solid sodium chloride, NaCl
(table salt) and solid sucrose, C12H22O11
(table sugar)
Safety:
If the conductivity tester used is powered by a 120v source, then use caution to prevent electric shock.
DO
NOT ALLOW STUDENTS TO USE THE APPARATUS.
If
you want students to test conductivity, obtain one or more
low voltage (battery powered) conductivity testers
The substances used are innocuous and do not
require special handling. Solutions may
be safely disposed of by flushing down the drain with water. The solid NaCl and
sugar may be used for all demonstrations and then disposed of in the trash.
Procedure:
Half-fill a 250mL beaker with solid NaCl and a second 250mL beaker with solid sucrose. Test the electrical conductivity of the solid
NaCl with the tester (light bulb remains dark). Clean
the electrodes and then test solid sucrose (light bulb remains dark). Ask students to verbalize and then record
what's observed in each case.
Half-fill
three 250mL beakers with distilled water.
Test the electrical conductivity of the distilled water in each of the
beakers (light bulb remains dark*).
Slowly add a substantial quantity of sugar to one of the beakers with
the conductivity tester in place. Stir
with a GLASS stirring rod until it’s clear that at least some of
the sugar has dissolved (light bulb remains dark*). Remove the conductivity tester from the first
beaker and rinse its contacts (wires) with distilled water. Place it in a second beaker and slowly add a
substantial quantity of salt. Stir with
a GLASS stirring rod until it’s clear that at least some of the
salt has dissolved (light bulb will gradually begin to glow as the salt
dissolves … finally becoming quite bright as the amount of dissolved salt
reaches a threshold level at which the full voltage is conducted through the
solution*). Remove the conductivity
tester from the second beaker and rinse its contacts (wires) with distilled
water. Place it in a third beaker
containing only distilled water. Ask
students to verbalize and then record what's observed in each case.
Analysis:
Referring
to the atomic structure of the sugar molecule and the salt formula unit, draw
out an inference from the students that, as the sodium and chloride ions (good
time to review cations & anions and what holds
them together in salt’s solid crystal lattice) accumulate, the electric current
is carried by the charged particles.
Sugar, a molecular substance, produces no charged particles and doesn’t
conduct electricity in solution.
* If your community water supply treats its tap water with chloroamines, any distilled water that you prepare on site will probably still conduct electricity. Use commercially prepared distilled or deionized water.
page 2
Magnetic Analogy for Bonding
Forces
Purpose:
This demonstration illustrates the nature of the
electrostatic attraction and repulsion between like and unlike charges using a
magnetic analog.
Materials:
·
4 pairs of ceramic ring or disk
magnets. In each pair, the parts need to
be different in size and/or appearance.
That is, they should be significantly different sizes … or significantly
different appearances (one solid & one with a hole in the center) … or,
better yet, both ! (2 pair is the minimum needed)
·
overhead projector and screen
Safety:
There are no special safety concerns.
Procedure:
On
the overhead projector, show that like poles repel and unlike poles attract as
follows:
ü
Set up four pairs of magnets so that the
different size and/or shape parts of the pair are attracted to each other. These pairs represent the attraction between
the nucleus and an electron. Move one
pair into the center of view. Then
approach with a second pair so that its "nucleus" approaches the
other "nucleus." No attraction
is observed. Move this pair so that the
"electrons" are between the two "nuclei." This leads to a
stable arrangement. If enough magnet
pairs are available, continue adding “nucleus-electron” pairs to show how the
positive and negative parts of the “atoms” attract/repel each other.
ü
With the stable arrangement in the center,
show that if either "electrons" or "nuclei" are forced
closer together, they repel. Thus an
"equilibrium distance" between particles with like charges is
created.
ü
Have students verbalize and then record
what's observed.
Practice
with the magnets prior to doing the demonstration. It requires patience to move the magnets
carefully to show formation of a "bond." Either ring or disk magnets may be used, but
the magnets should be face-magnetized.
That is, the faces should be the poles.
It's possible to use ring magnets to represent the nuclei and disk
magnets to represent the electrons. In
either case, use large diameter magnets for nuclei and small diameter magnets
for electrons.
Analysis:
Referring
to atomic structure, .point out that this is analogous to the electrostatic
forces that cause an ionic bond to form.
Both magnetic and electrostatic forces behave the same way; like charges
or poles repel and unlike ones attract.
Point out that a "nucleus-electron" pair is stable because
there's only one attractive force and no repulsive forces. When two pairs
approach, new attractive forces arise between the "nucleus" of one
pair and the "electron" of the other pair, and vice versa. New repulsions also are present between the
two "nuclei" and the two "electrons." The result is four attractions but only two repulsions, hence the two pairs form a stable arrangement.
page 3
Force at a Distance
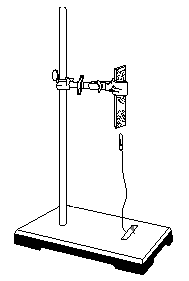 This simple demonstration shows that forces
can exist even though objects are not in direct contact.
This simple demonstration shows that forces
can exist even though objects are not in direct contact.
A paper clip is attached to a fine thread or
long hair. The other end of the thread
or hair is taped to the base of the stand.
Adjust the length of the thread or hair so that there is a small gap
between the magnet and the paper clip.
A piece of paper can be passed between the magnet and the paper clip to
show that there is no direct, physical connection between the two.
Figure 4. Force
between a magnet and a paper clip.
page 4
Physical Properties and
Chemical Bonding in Solids
Note: This is appropriate for use
as a student activity or a demonstration, either student or teacher.
Purpose:
This activity enables students to “see” how the atoms in solids bond
together to form chemical bonds.
Materials:
Styrofoam balls (preferably different colors and sizes … blocks could be
used as well), toothpicks and contact cement.
Procedure:
Establish the “ground rules” first …
ü
toothpicks
represent electrons available to be shared
ü
contact cement
represents the electrostatic attraction between charged particles formed by
giving up (cations) or accepting (anions) outer shell
electrons.
Using styrofoam balls of different colors
and/or sizes (use different shapes if balls are not available, or if you want
to add variety), have students prepare models of atoms that form cations & anions (such as the alkali metals, alkaline
earth metals and the halogens) by coating the top, bottom, front, back, right
& left sides with contact cement.
Then have students prepare models of atoms that typically share
electrons by placing toothpicks at points on the styrofoam where chemical bonds will form as a result
of electron sharing.
Have the students assemble the ionic crystal lattice. If you haven’t covered the types of crystal
lattices, confine your work to the face-centered cubic style illustrated by NaCl. As they
assemble the model atoms, the contact cement will form a tight bond between the
ions. As they assemble the shared
electron compounds (water & methane are good examples) they should stack
the molecules in a pile to represent a sample of the material in its solid
state.
Have students separate the molecules of the compound(s) they’ve created
to represent the amount of effort (energy) needed to melt the material.
Analysis:
Since the contact cement is analogous to the electrostatic forces in
ionic crystals and the individual molecules piled together are indicative of
the bonding between molecules, the contrasting difficulty of separating the
ions as opposed to the molecules represents the energy required to change the
material from its solid to its liquid state.
page 5
Metals and Ionic
Note: This is appropriate for use
as a student activity or a demonstration, either student or teacher.
Purpose:
This activity enables students to “see” how the electron distribution in
ionic crystals and metals affects their physical properties.
Materials:
Styrofoam balls (preferably different colors and sizes … blocks could be
used as well), gooey stuff (peanut butter works well) and contact cement.
Procedure:
Establish the “ground rules” first …
ü
the gooey stuff
represents the delocalized arrangement of electrons found in metals.
ü
contact cement
represents the electrostatic attraction between charged particles formed by
giving up (cations) or accepting (anions) outer shell
electrons.
Using styrofoam balls of different colors
and/or sizes (use different shapes if balls are not available, or if you want
to add variety), have students prepare models of atoms that form cations & anions (such as the alkali metals, alkaline
earth metals and the halogens) by coating the top, bottom, front, back, right
& left sides with contact cement.
Then have students prepare models of metallic atoms by coating the styrofoam balls with gooey stuff
(use a lot and the physical properties of malleability and ductility will come
across clearly). If you use only one
kind of styrofoam ball, your
model will be that of a metallic element whereas more than one kind will
represent a metal alloy.
Have the students assemble the ionic crystal lattice. If you haven’t covered the types of crystal
lattices, confine your work to the face-centered cubic style illustrated by NaCl. As they
assemble the model atoms, the contact cement will form a tight bond between the
ions. Then have students assemble
metallic atoms by sticking them together with gooey stuff (use a lot and the
physical properties of malleability and ductility will come across clearly) …
just stick ‘em together any ol’
way.
Malleability: Have
students test the models for malleability by gently applying pressure to the
assembled atoms. The ionic crystals will
resist changing shape until the pressure reaches a point at which the
electrostatic bonds are broken and the crystal shatters. The metallic atoms will flow past one another
‘til they form a single layer.
Ductility: Have students push the mass of
assembled atoms through an opening that’s smaller than the overall size of the
mass of assembled atoms. The metallic
atoms will reorganize and squeeze through whereas the ionic crystal will resist
going through intact.
Analysis:
Since the contact cement is analogous to the electrostatic forces in
ionic crystals and the gooey stuff is indicative of the delocalized electrons
in a metal, the ease of rearranging metallic atoms as contrasted with the
resistance to rearrangement of the ions in a crystal illustrates their
contrasting malleability and ductility.
page 6
Combining Cations & Anions Using Model Ions
Note: This is appropriate for use
as a student activity or a demonstration, either student or teacher.
Purpose:
This activity enables students to “see” how positively charged ions
(electron donors or cations) and negatively charged
ions (electron acceptors or anions) combine in certain ratios to form
compounds.
Materials:
Blank paper & small sticky notes (Post-its).
Procedure:
Establish the “ground rules” first …
ü
the paper rectangle
with the ion’s (monatomic or polyatomic) symbol represents all of the atom (or
combination of atoms in the case of polyatomic ions) except for the outer shell
or valence electrons.
ü
sticky notes
represent the outer shell or valence electrons.
ü
a paper rectangle
with no sticky notes (because they’ve been donated to another atom) has a
filled outer shell (the shell that was previously just below the unfilled outer
shell).
ü
the number of
sticky notes moved represents the amount of energy required to make the atoms
combine.
ü
each electron
accepted increases an atom’s negative charge by one.
ü
each electron
donated increases an atom’s positive charge by one.
Have students tear a sheet of paper into 16 pieces. On each of these, put the symbol of a
representative element. (Teacher
Note: Use your judgment regarding which elements and/or polyatomic ions you
include)
Attach sticky notes to each “atom” according to the number of electrons
in its outer shell.
Place two model atoms side-by-side (Na & Cl
are good to start with). Transfer
electrons from one atom to the other with the least expenditure of energy (see
ground rules above). If one of the
species of atom cannot donate enough electrons or accept enough electrons so
that all the atoms end up with a filled outer shell, add more atoms of the
appropriate species ‘til, by donating and accepting electrons, all atoms have a
filled outer shell. The paper rectangle represent the formula unit of the ionic compound
formed.
Analysis:
Metals attain noble gas electron configuration by donating outer shell
electrons ‘til the outer shell is empty and the shell immediately below, which
is full, becomes the outer shell.
Non-metals accept electrons ‘til their outer shell is filled and they
achieve noble gas electron configuration.
Noble gas electron configuration is stable.
page 7
Key Questions
1.
What are valence electrons
?
Electrons in the highest
occupied energy level of an atom are known as valence electrons. These
electrons determine what kind of chemical bonds, if any, the atom can form.
2.
How can the total valence electrons for an
element be determined ?
Electron configurations
may be used to determine the number of valence electrons for an element. For
example, hydrogen has only one electron-its valence electron. The configuration
is 1s1. For representative elements, the number of valence electrons equals the
total electron population at the highest principal energy level (n), as
indicated by electron configurations.
3.
How many valence electrons does a sodium,
silicon, beryllium, and oxygen atom have ?
sodium
= 1; silicon = 4; beryllium = 2; oxygen = 6
4.
What is the relation between the number of
valence electrons in atoms of an element and the element's placement in the
periodic table ?
The number of valence
electrons determines the group placement of an element. For example, hydrogen
has one valence electron; it's in the alkali metal family. All other elements
in this family, Li, Na, K, Rb, and Cs, also have only
one valence electron. On the other hand, fluorine has seven valence electrons,
as shown by its configuration 1s2 2s2 2p5.
This places it in the halogen family.
5.
Draw orbital diagrams for atoms of sodium
and fluorine. Use the diagrams to write Lewis-dot formulas for these elements.
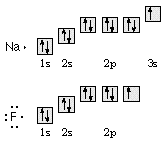
6.
How does the periodic table help to
determine the number of valence electrons for an element ?
Traditional group numbers (at least as commonly used in
the
7.
Why are molecules more stable than
separated atoms, particularly among representative elements ?
The origin of chemical bond stability depends upon the attractive and repulsive electrostatic forces present. Electron-nucleus interaction always furnishes attractive forces while nucleus-nucleus and electron-electron interactions furnish repulsive forces. A simple way to show the origin of this stability is in terms of possible attraction for one or more electrons simultaneously by two nuclei close to each other. Whether two given atoms can form a bond depends upon the filling of orbitals in the separate atoms. Figure 6 illustrates this concept. The hydrogen molecule is used to show that when two hydrogen atoms are close together, there's a possibility of more attractive forces than repulsive forces. On the other hand, when two helium atoms approach, a net attractive force does not occur, although there are four new attractive forces. This can be explained … at least in terms of this simple analysis … by the equal number of new repulsive forces also formed, as shown in Figure 6.
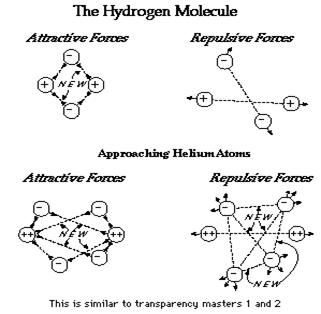
Figure
6. Bonding of the hydrogen
molecule.
8. How many bonds can be formed by atoms of
representative elements ?
The number of bonds that an
atom of a representative element can form depends upon the orbital occupancy of
valence electrons in the atom. Beginning with the halogen family, the valence
electron shell has seven electrons, three pairs and one unpaired electron in s
and p orbitals … for example, 3s2 3px2
3py2 3pz1 in chlorine.
This enables any halogen element to form a single covalent bond by
forming one electron pair with an unpaired valence electron from another
atom. This is shown by the formation of
HF, illustrated in Figure 7.
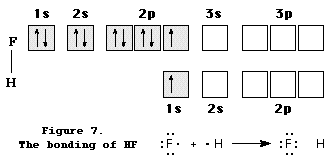
In the case of oxygen family
elements, each element's atom has six valence electrons. Two of these are unpaired … for example, 2s2
2px2 2py1 2pz1 in oxygen. Two single covalent bonds, as for oxygen in
water, may be formed under these circumstances, as illustrated in Figure 8.
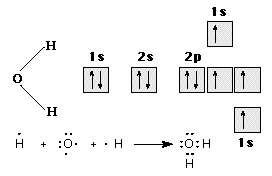
Figure 8: The Bonding in H2O
9. What is the octet rule ?
When atoms react, they often
change electron populations to acquire the stable electron configuration of a
noble gas. That is, eight electrons in
the outer energy level. For hydrogen, of
course, this would be a "duet" rule with two hydrogen atoms sharing
two electrons. This is not a hard &
fast rule since there are many exceptions.
It is useful in predicting the bonding expected when many atoms form
compounds. If the term "octet
rule" is objectionable, an alternative is to point out that atoms tend to
seek noble gas electron configurations either by electron sharing (covalent
bonding) or transferring (ionic bonding) when forming compounds.
10. In chemical reactions, do metals and
nonmetals behave the same or differently with respect to sharing or
transferring electrons ?
Metals generally have lower electronegativities than do nonmetals. Thus, metal atoms attract electrons less
strongly and tend to lose electrons to acquire an octet (noble gas electron
configuration). This gives the metal
atom a net positive charge, resulting in a cation. Nonmetals, on the other hand, behave in the
opposite manner, having higher electronegativities than
metals. Nonmetal atoms tend to gain
electrons to acquire a noble gas electron configuration, giving them a net
negative electric charge. Nonmetals tend
to gain electrons and form negatively-charged ions (anions). These generalities hold reasonably well for
many reactions involving representative elements, particularly if higher
members of the carbon family are excluded.
11. Draw orbital diagrams for the sodium ion, Na+,
and the chloride ion, Cl-, showing the
outermost energy level only. Write the
Lewis-dot formulas and electron configurations for these species.
Teacher's Note: Any simple monatomic ion will work with
this question, For
example, Li+, F-, Mg2+, S2-,
etc. Lewis-dot formulas of single ions
are usually enclosed in brackets and the ionic charge indicated.
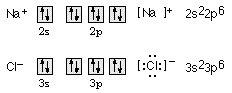
12. Draw Lewis-dot structures for NH3,
H2O, Cl2O, C2H4, and SiO2.
Teacher's Note: Many compounds may be used for this
question. Avoid "problem"
molecules such as those with an odd number of valence electrons that cannot
follow the octet rule (such as NO), at least initially. When teaching students how to draw Lewis-dot
structures, a useful technique is to develop a helpful set of rules, such as
these:
a. Count
the total number of valence electrons in the structure.
b. For
ions, add one electron for each negative charge and subtract one electron for
each positive charge.
c. Draw
the skeleton using dashes to represent electron pairs joining two atoms
together until the skeleton is complete.
d. Add
dot-pairs until all valence electrons are accounted for and each atom has an
octet of electrons (duet for hydrogen).
e. If Step d is impossible when
N, C, O, or S are involved, try double or triple bonds (two pairs or three
pairs of dots) to form octets.
13. Compare the covalent bonds formed between
elements of similar electronegativity such as carbon
and hydrogen and covalent bonds formed between elements with significantly different electronegativity,
such as hydrogen and chlorine.
Student answers will probably
vary considerably. At minimum, some
reference to equal or unequal electron sharing should be made. Students should note that unequal sharing
produces a charge separation. In any
case, students should point out that polar covalent bonds, depending on
molecular geometry, often give a molecule properties
that affect its behavior.
14. Describe the bonding trend expected when
fluorine bonds with each element in the second row of the periodic table,
including itself … F2, OF2, NF3, CF4,
BF3, BeF2, and LiF.
Students should recognize
that a variation in bond type from homonuclear
covalent to essentially ionic takes place.
In their answers, students may note the arbitrary nature of deciding at
which point polar covalent bonds are better regarded as ionic.
15. What happens to the system's total potential
energy when two isolated atoms capable of bonding come into close proximity ?
As atoms approach, the atomic
nuclei are attracted to the valence electrons.
If both atoms have half-filled or empty valence orbitals,
then bonding may occur, lowering the potential energy of the system due to
attractive forces reducing the separation between atoms. See Figure 11.
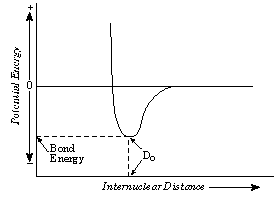
16. Metals have characteristic properties that can
be explained in terms of bonding.
Briefly describe the bonding in metals that explains such properties.
The basic ideas are
illustrated in the lab involving chemical bonding in solids found on page 4.
17. Some substances are molecular with low melting
points, soft structure, and low solubility in water. What kind of bonding could account for these properties ?
It's probable that students
will not give complete answers without help from you. Three types of intermolecular interactions
(not including the strong covalent bonding within the molecules themselves) may
be responsible. These interactions are
collectively referred to as van der Wals forces. The
weakest intermolecular forces are
Another type of weak
intermolecular bonding force is a dipole-dipole force. Two kinds of these forces, distinguished by
the energy required to break them, are possible. One is the attractive force between opposite
charges of polar molecules. It is
stronger than
Some compounds appear to have
abnormally high melting points when compared to compounds of similar size,
shape, and total electrons. In many of
these cases, such compounds exhibit hydrogen bonding, involving highly
electronegative nitrogen, oxygen, or fluorine atoms. The small size and high electronegativity
of such atoms cause highly unequal sharing of the electron pair forming the
covalent bond to hydrogen; there is substantial separation of charge. The intermolecular attractions (between the
hydrogen atom and a lone pair of electrons on a N, O,
or F atom from an adjacent molecule) arising in this fashion are about an order
of magnitude stronger than ordinary dipole-dipole bonding. Water is an example of a hydrogen-bonded
substance. If its intermolecular forces
were simple dipole-dipole forces, it would melt and boil at lower temperatures
than hydrogen sulfide, H2S, which is a gas at room temperature.
page 8
Rubric for Teacher Observation & Evaluation
Under Development
page 9
Rubrics for Lab Write-ups
Under Development
page 10
Written Quizzes & Tests
Under Development