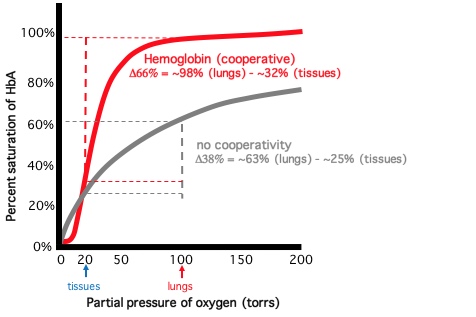
| Percent oxygen saturation as a function of oxygen partial pressure (torrs) is displayed below for Hemoglobin (HbA) and a theoretical non-cooperative oxygen transport protein (NC). The sigmoidal, cooperative curve for HbA shows that the percent saturation of HbA increases or decreases in a non-linear fashion, depending on the oxygen partial pressure. This sigmoidal curve results from the allosteric binding of oxygen that regulates HbA subunit interaction and oxygen affinity. Compare the percent saturation of HbA and NC at ~100 torrs (lung partial pressure of oxygen): ~98% vs ~63%. Now compare the percent saturation of both proteins at ~20 torrs (tissue partial pressure of oxygen): ~32% vs ~25%. Thus, the % saturation change going from lung partial pressures to tissue partial pressures is ~1.7 times greater for HbA than NC (Δ66% vs Δ38%). This calculation demonstrates the functional consequence of cooperative oxygen binding/release by HbA: this remarkable protein can deliver 1.7X more oxygen than if binding/release of oxygen by HbA subunits were independent of one another. |
