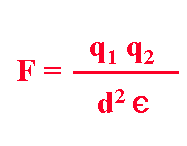back
to pol III beta subunit exhibit
Ionic
bonds are electrostatic
attractions between oppositely charged species. The strength of an ionic bond
is related to distance: ionic bonds are not "broken", their strength
is simply diminished by distance. Ionic bonds may be formed at any angle, since
electrons are not shared covalently. Thus, variable arrangements of ionic bonds
are found in different contexts. The strength of an ionic bond is given by:
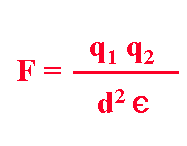
Experiments
show that bond strength (F) is directly proportional to the amount of charge
on each interacting atom (q), is inversely proportional to the distance (d)
between them, and is inversely related to e, the dielectric constant
of solvent. The value of the dielectric constant (a measure of the capacity
of solvent molecules to neutralize charge by reorientation) is correlated
with solvent polarity.
Due to the inverse relationship
between ionic bond strength and e, the local environment of an ionic
bond largely determines its strength. If an ionic bond is surrounded by polar
H2O molecules(e=85), the charges of the interacting molecules
are effectively reduced by interaction with these H2O shells, and
the strength of the bond is correspondingly reduced. However, if an ionic bond
is inaccessible to solvent, as in the interior of a protein, the charged species
are mostly surrounded by hydrophobic side chains with low e's. For example,
for CH3, e=1. Thus, ionic bonds that are not accessible to solvent (H2O)
tend to be quite strong, even approaching that of a covalent bond (~50 kcal/mole).
back
to pol III beta subunit exhibit