 Inorganic
Chemistry
Inorganic
ChemistryBODY CHEMISTRY
Chapter
2
 Inorganic
Chemistry
Inorganic
Chemistry
Elements: basic substances
that cannot be broken down into simpler substances by ordinary chemical means.
Atoms of elements combine chemically to form molecules.
Compounds are composed of molecules with more than one kind of element linked
by chemical bonds.
Bonds
Ionic -- attraction of opposite charges (+ and -)
Covalent--sharing of one or more pairs of electrons. Stable. They link elements
in small and large compounds.
Hydrogen--formed when the hydrogen atom, already covalently bonded in a compound,
acquires a slight positive charge and becomes attracted to negatively charged
atoms. Hydrogen bonds are responsible for the association of most organic molecules
and water.
Common Elements (Know these!)
Chemical Reactions
Combining or breaking apart two or more atoms that brings about a chemical change
is called a chemical reaction.
Metabolism: includes all the chemical reactions that occur in the body.
Anabolism: building-up metabolic reactions. "Anabolic steroids."
Catabolism: tearing-down metabolic reactions.
Hydrolysis and Condensation Hydrolysis: water broken down in the process.
Condensation: water produced as byproduct of process.
Animations
of Hydrolysis and Condensation
Water Makes up 62% of the body by weight
Structure: a "polar" molecule. Forms hydrogen bonds. Primary solvent of life.
Transporter -- nutrients, wastes, gases are transported via water.
Temperature Regulator -- high heat capacity
Lubricant -- joints, digestive tract
Acids, Bases, Salts,
& Buffers
Acid: releases hydrogen ions (accepts hydroxyl ions)
Base: releases hydroxyl ions (accepts hydrogen ions)
Salt: compound formed during neutralization reaction pH scale: measures the
degree of acidity or alkalinity of a solution--a measure of the number of free
hydrogen ions. The pH scale is a logarithmic scale, like the Richter scale.
Each step increase represents a ten-fold difference in hydrogen ions.
Buffers: salts + weak acid or base. Buffers resist changes in pH Carbonate and
phosphate buffers are important in regulation of body fluid pH.
ChemTutor: Compounds
Organic
Compounds
Organic compounds always contain carbon and hydrogen.
Common “Optional elements” are oxygen, nitrogen, and sulfur.
Carbohydrates
General formula is CH2O or CnH2nOn
Functions:
Saccharides are subunits:
 Glucose
(© IconBazaar, limited permission)
Glucose
(© IconBazaar, limited permission)
Note: black spheres represent carbon, red spheres oxygen and white spheres hydrogen.
Lipids
Fatty acids: chains of CH2 groups with a terminal carboxylic acid. Basic
building blocks of most complex lipids.
Saturated: no double bonds;
"saturated" with hydrogen. Saturated fats are solids at room temperature.
Example: palmitate
Unsaturated: one or more
C=C double bonds (loss of hydrogen). Generally liquid at room temperature.
Example: oleate
Polyunsaturated and Monounsaturated oils.
Not all unsaturated fats are the same. Cis fatty acids are more fluid than trans fatty acids.
Some simple fatty acid amides can act as hormones -- e.g., recently identified sleep inducing hormone.
Triglycerides: glycerol linked to 3 fatty acids
Phospholipids: 2 fatty acids linked to glycerol + phosphate group + alcohol.
Phospholipids are "bipolar" -- compound has a charged end and uncharged end.
Phospholipids make up most of cell membranes.
Steroids: Fats in ring form. In cell membranes; cholesterol, hormones.
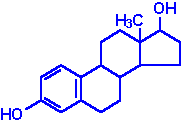 Estradiol
(Estrogen)
Estradiol
(Estrogen)  Testosterone
Testosterone
Eicosanoids: Most abundant are prostaglandins -- prostaglandins are typically
"local hormones" since they are involved in local reactions such as smooth muscle
contraction (e.g., labor), mucus production in the stomach, blood clotting,
and inflammation.
Aspirin inhibits prostaglandin synthesis.
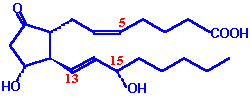 Prostaglandin E
Prostaglandin E
Proteins
Large complex molecules composed of subunits called amino acids
Amino: amine group --NH2.
Acid: carboxyl group -- COOH.
"R" group is different in the 20 common amino acids that make up proteins.
Amino acids are joined together by peptide bonds (via condensation reaction)
Amino acids linked by peptide bonds are called Peptides.
2 linked amino acids = dipeptide,
3 = tripeptide,
4 or more = polypeptide.
Levels of Protein organization
Primary -- linear arrangement of amino acids.
Secondary -- interactions between differ amino acids (hydrogen bonds) result
in the formation of helices and sheets.
Tertiary -- interactions between different parts of a helix cause the protein
to fold in different ways.
Quaternary -- separate helices interact forming complex structures; form taken
can mean the difference between active and inactive proteins.
Protein types: Globular and fibrous.
Change in a single amino acid can result in a cascade of changes in protein structure -- Sickle cell anemia would be an example of how such a subtle change causes a serious disease.
Substitution of one amino acid in beta-hemoglobin chain (charged glutamic acid replaced by neutral valine) causes hemoglobin to form rod-like filaments when oxygen is low. The filaments cause red blood cells to sickle. Sickled cells block small capillaries and the spleen works overtime removing abnormal cells.
Functional Classifications
Structural, contractile, transport, buffers, enzymes, coordination (hormones),
and antibodies.
Enzymes: act as catalysts for reactions -- they are organic catalysts.
Enzyme nomenclature: -ase
suffix is common, e.g., protease breaks down protein, lipase breaks down lipids.
" -in" suffix was used for some of the first enzymes characterized,
e.g., pepsin, trypsin since "-in" was the common suffix for proteins.
Enzymes may require cofactors or coenzymes to function properly -- common examples
of cofactors are magnesium, calcium, zinc. Many vitamins are coenzymes
Enzymes have important technological uses: Glucose-test strips and meters along
with Industrial enzymes. Recombinant DNA technology/Biotechnology
Protein Hormones
Insulin:
Globular protein
Pig
insulin in 3D
Nucleic
Acids
2 types: deoxyribonucleic acid (DNA) and ribonucleic acid (RNA) DNA and RNA
differ in the type of simple sugar they have -- deoxyribose or ribose. They
also differ in structure and function.
Subunits are nucleotides:
Nucleotide = Base + sugar + phosphate
Adjacent nucleotides are linked together via sugar-phosphate bonds, making a
"backbone" for the large molecule 5 different bases: Adenine (A) Guanine (G)
Thymine (T): only in DNA Cytosine (C) Uracil (U): only in RNA
Other nucleotides: ATP (adenosine
triphosphate) and GTP have "high energy" phosphate bonds. Used as
energy sources in the cell -- "cell money."
ATP ---> ADP + P + energy (The terminal high energy phosphate bond of ATP is
broken, producing adenosine diphosphate, a free phosphate, plus free energy
which can then be used to power other reactions in the cell).
DNA ![]()
DNA is the genetic code of most organisms -- its structure is the code for the
different proteins that an organism manufactures. Double helix -- the double
helix is the basic form of DNA. Two strands of DNA are held together by hydrogen
bonds which form between the base (base
pairing). Adenine pairs with thymine, cytosine pairs with guanine.
The sequence of bases is a linear code.....
one strand: -A-T-G-C-C-G-T-A-A-T-T-C-G-A-T-
opposite strand: -T-A-C-G-G-C-A-T-T-A-A-C-C-T-A-
A chromosome
is condensed chromatin, a collection of proteins and DNA (many genes).
A gene
is a sequence of nucleotides which the cell can decode to form a protein (or
a non-translated form of RNA) -- details to be covered in next chapter.
DNA serves as a template for RNA. "DNA codes for RNA"
one strand DNA: A-T-G-C-C-G-T-A-A-G-T-C-G-A-T
complementary RNA: U-A-C-G-G-C-A-U-U-C-A-G-C-U-A
DNA is found in the nucleus, but proteins are formed in the cytoplasm. Genetic
code is transferred from the nucleus to cytoplasm via RNA.
RNA serves as the direct code for protein synthesis. RNA is single stranded whereas DNA is double stranded.
Miscellaneous links
Large Molecules Problem Set: Take the Quiz! This is an excellent site that includes a tutorial on macromolecules.
CHEMystery Site: Organic chemistry overview -- Check out "Biochemical" links.
Left-Handed DNA Hall of Fame: lots of neat DNA images