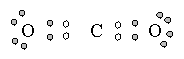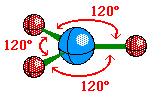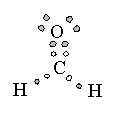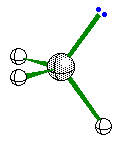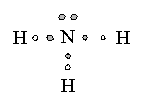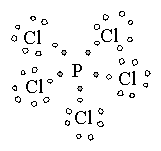
In Valence Shell Electron Pair Repulsion (VSEPR) theory, pairs of electrons that surround the central atom of a molecule or ion are arranged as far apart as possible to minimize electron-electron repulsion. This simple idea can be used to predict the shapes of molecules by following a simple procedure
· Decide which is the central atom in a molecule.
- In cases of ambiguity, pick the least electronegative atom as this atom will be better able to share its electrons with the other atoms in the molecule.
· Count up the valence (outer shell) electrons on the central atom.
· Count up the electrons used by the outer atoms to make bonds with the central atom.
· The sum of (2) + (3) divided by two gives the Valence Shell Electron Pair (VSEP) count.
· The predicted geometry of the molecule is based on the number of VSEP.
|
VSEPR |
Shape |
|
Example |
Hybridization |
|
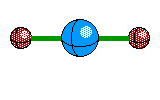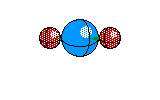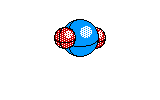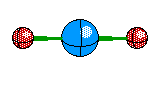
2 |
Linear |
|
Carbon dioxide |
sp hybridization |
|
3 |
Trigonal Planar |
|
Formaldehyde |
sp2 hybridization |
|
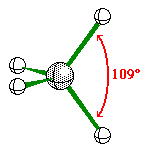
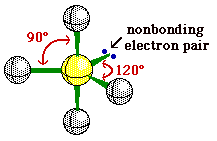
4 |
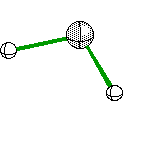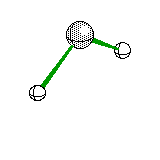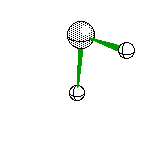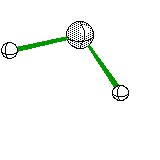
Trigonal Pyramidal |
|
Ammonia |
sp3hybridization |
|
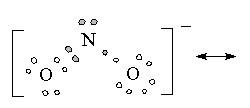
5 |
Bent |
|
Nitrate ion |
sp2 hybrization |
|
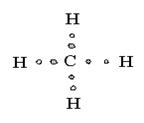
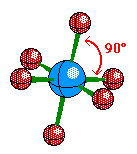
6 |
Tetrahedral |
|
Methane |
sp3 hybridization |
|
7 |
Trigonal Bipyramidal |
|
Phosphorus pentachloride |
sp3d1 hybridization |
|
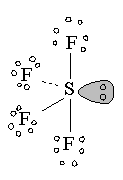
8 |
SeeSaw |
|
Sulfur tetrafluroide |
sp3d1 hybridization |
|
9 |
Octahedral |
|
Sulfur hexaflouride |
sp3d2
hybridization |
For a comparison of these shapes
click on the following link. 
The following chart summarizes the
shapes and hybridization of certain compounds
|
Molecule |
# of electron pairs |
Shapes with, and without non-bonding e pair |
Hybridization of central atom |
|
BeH2 |
2 |
linear, linear |
sp |
|
BF3 |
3 |
trigonal planar, trigonal planar |
sp2 |
|
CH4 |
4 |
tetrahedral, tetrahedral |
sp3 |
|
NH3 |
4 |
tetrahedral, trigonal pyramidal |
sp3 |
|
H2S |
4 |
tetrahedral, bent |
sp3 |
|
PF5 |
5 |
trigonal bipyramidal, trigonal bipyramidal |
dsp3 |
|
BrF3 |
5 |
trigonal bipyramidal, T-shaped |
dsp3 |
|
TeCl4 |
5 |
trigonal bipyramidal, Seesaw |
dsp3 |
|
SF6 |
6 |
octahedral, octehedral |
d2sp3 |
|
XeF4 |
6 |
octahedral, square planar |
d2sp3 |
|
XeF2 |
5 |
trigonal bipyramidal, linear |
dsp3 |
Click on the link below to test your
knowledge of molecule shapes and hybridization
Click on the link below for a paper
and pencil quiz on the V.S.E.P.R